- OCTG & Line Pipe
- Fittings
- Flanges
- Valves
- Rig Components & Tools
- Chemical Products
- Glacial Acetic Acid
- Toluene
- Xylene
- Acetone
- Ammonia Water
- Caustic Soda Liquid
- Cyclohexanone
- Dimethylformamide
- Epichlorohydrin
- Ethyl Acetate
- Formaldehyde
- Formic Acid
- Hydrochloric Acid
- Hydrogen Peroxide
- Methanol
- Methyl Ethyl Ketone
- Methyl Isobutyl Ketone
- Methylene Chloride
- N-butyl Acetate
- Nitric Acid
- Phenol
- Sulphuric Acid
- Trichloroethylene
- Personal Protective Equipment
Dimethylformamide

Dimethylformamide
Dimethylformamide is an organic compound with the formula (CH3)2NC(O)H . Commonly abbreviated as DMF (though this acronym is sometimes used for dimethylfuran), this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Pure dimethylformamide is odorless whereas technical grade or degraded dimethylformamide often has a fishy smell due to impurity of dimethylamine. Its name is derived from the fact that it is a derivative of formamide, the amide of formic acid. Dimethylformamide is a polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such as SN2 reactions. Dimethylformamide can be synthesized from methyl formate and dimethylamine or by reaction of dimethylamine with carbon monoxide. Dimethylformamide is not stable in the presence of strong bases like sodium hydroxide or strong acids such as hydrochloric acid or sulfuric acid and is hydrolyzed back into formic acid and dimethylamine, especially at elevated temperatures.
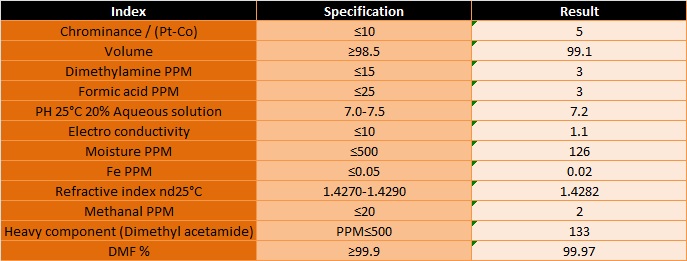
Product Identification
CAS NO : 68-12-2
Molecular Formula: (CH3)2NC(O)H
Molecular Formula: (CH3)2NC(O)H
Hazard Class: 3 (Packing : 3)
Specifications